Collaboration: K. Sengupta (CINaM), Y. Hamon (CIML), R. Varma (NIH, USA), JT Groves (U. Berkeley, USA), T. Fiodelisio-Coll (UNAM, Mexico)
Fluid and nanopatterned substrates
We interrogate T lymphocyte physiology by designing carefully controlled artificial surfaces to stimulate cell activation and spreading. Our original artificial surfaces exhibit fixed or mobile ligands, and arrays of submicrometric adhesive patches surrounded with repulsive zones (Nanoletters 2013, 2015). These surfaces are compatible with high resolution surface microscopy like TIRFM and RICM. With these tools, we have demonstrated that (1) ligand mobility controls lymphocyte spreading and we proposed a physical mechanism based on friction to explain these results (Biophys. J. 2014), (2) cell adhesion area is determined by global ligand density, but local cell-substrate interface and receptor clustering is tuned by underlying adhesive patch geometry (Integr. Biol. 2016). We have also found a non-monotonous response of spreading area to substrate rigidity, T-cell spreading mediated by anti-CD3 being maximal for a substrate rigidity of around 50 kPa.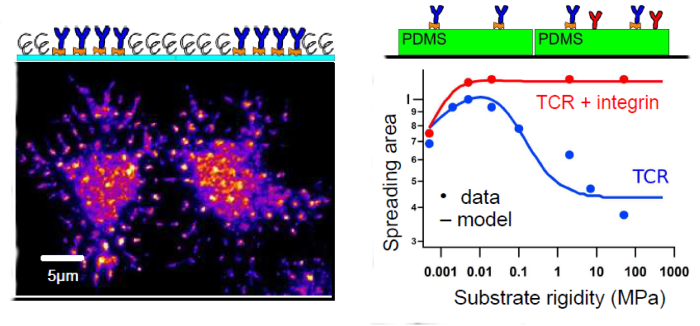
Fig. Examples of T-cell spreading on nanopatterned substrates (left; TCR labelled) and substrates of variable rigidity (right; adhesion via TCR only or TCR+Integrin)
Shaping cells to control their activation and mechanics
We introduced the use of “shaped T cells“ during the thesis of A. Sadoun, since the spreading state of cells, on any adhesive molecule, is strongly inhomogeneous, giving rise to strong dispersion of the mechanical parameters at population levels . The cells were adhered on micro-stamped adhesive / repulsive patterns that will impose given levels of adhesion and cell shapes. The design of the patterning strategy was crucial since the T cells are small (~10 µm), and extremely activable, so required specific passivation strategies : using an advanced calcium imaging processing technique at cell scale, we determined the best adhesive / repulsive molecular combination, the most favourable concentration conditions and cell densities, in order to achieve different patterned, non activated but activable T cells. We also obtained patterned model APCs (transfected COS-7 cells, see below). All cells were characterized mechanically using AFM indentation. We and others have established that APC like cells (monocytes, macrophages, COS-7) are one order of magnitude stiffer (~1kPa) than typical T cells (~100Pa). We quantified, using RICM and confocal microscopies the adhesion and structures of the patterned cells (membrane, cytoskeleton, nucleus) and their activation by measureing their calcium fluxes when submitted to soluble antiCD3 or sedimenting model APCs. As such, we established a platform of standardized T cells for mechano-activation studies. We also studied the structure and interaction of immune cells with standardized APC cells, which we caracterized using a collection of optical microscopies, surface microscopies and AFM imaging and force modes.
In the frame of the FR/MEX IRP BioPhysImmuno (Coord. PH Puech)
References
Dillard P, Varma R, Sengupta K, Limozin L (2014) Biophys. J. 107:2629-2638. Pubmed
Dillard P, Pi F, Lellouch AC, Limozin L, Sengupta K (2016) . Integrative Biology 8:287. Pubmed
Wahl A, Dinet C, Dillard P, Puech P-H, Limozin L, Sengupta S (2019) PNAS 116:5908-5913. Pubmed
Dejardin MJ, Hammerle A, Sadoun A, Hamon Y, Puech PH, Sengupta K, Limozin L Lamellipod reconstruction by three dimensional reflection interference contrast nanoscopy (3D-RICN). Nanoletters (2018) Oct DOI: 10.1021/acs.nanolett.8b03134
A. Sadoun, M. Biarnes-Pelicot, L. Ghesquiere-Dierickx, A. Wu, O. Théodoly, L. Limozin, Y. Hamon, P.-H. Puech, Controlling T cells spreading, mechanics and activation by micropatterning. Sci Rep. 2021 Mar 24;11(1):6783. https://doi.org/10.1038/s41598-021-86133-1
Sengupta K, Dillard P, Limozin L., 2024. Morphodynamics of T-lymphocytes: Scanning to spreading. Biophysical Journal. https://doi.org/10.1016/j.bpj.2024.02.023

You must be logged in to post a comment.