A new commentary has been published in the EMBO journal called “Unraveling T-cell decoding strategy : a step forward” by Philippe Robert & Pierre Bongrand.
This invited commentary provided us with an opportunity to summarize the present stage of a long-term quest for the unraveling of T lymphocyte decision-making after encountering a foreign antigen.
This problem is of both practical and theoretical importance. Firstly, understanding cell behavior is a major challenge for cell biologists and this active research field involves “omic” approaches, biophysics, and theoretical modeling with or without artificial intelligence tools. Secondly, the growing importance of immunotherapy is dependent on the choice of target antigens used for immunization or Car-T-cells generation.
A number of reports published three decades ago strongly supported the view that T cell decision could be predicted by conventional descriptors of the interaction between T cell receptors and antigen ligands, such as affinity constants or kinetic rates measured on soluble ligands and recepors. However, despite a general agreement with experience, some discrepancies remained.
During the following years, studies performed at the single molecule level by a number of biophysically oriented teams, including LAI investigators, clearly demonstrated that aforementioned descriptors did not satisfactorily account for the interactions between membrane-bound molecules, due to the influence of mechanical forces, intervening molecules, and limitation of molecular motions. A number of experimental reports suggested that aforementioned discrepancies could be due to mechanical effects.
In the current issue of the Embo Journal, a report suggests that these discrepancies between T cell activation and measured affinity constant were in fact due to a faulty affinity determination. The authors conclude that the conventional affinity constant may be the best predictor of T cell decision.
We suggested in our commentary that this important result was not in contradiction with the current view that the detailed mechanisms of T cell activation are dependent on the fine properties of ligand binding and that further studies are needed to build an exhaustive model of the activation process.
https://doi.org/10.1038/s44318-025-00645-4
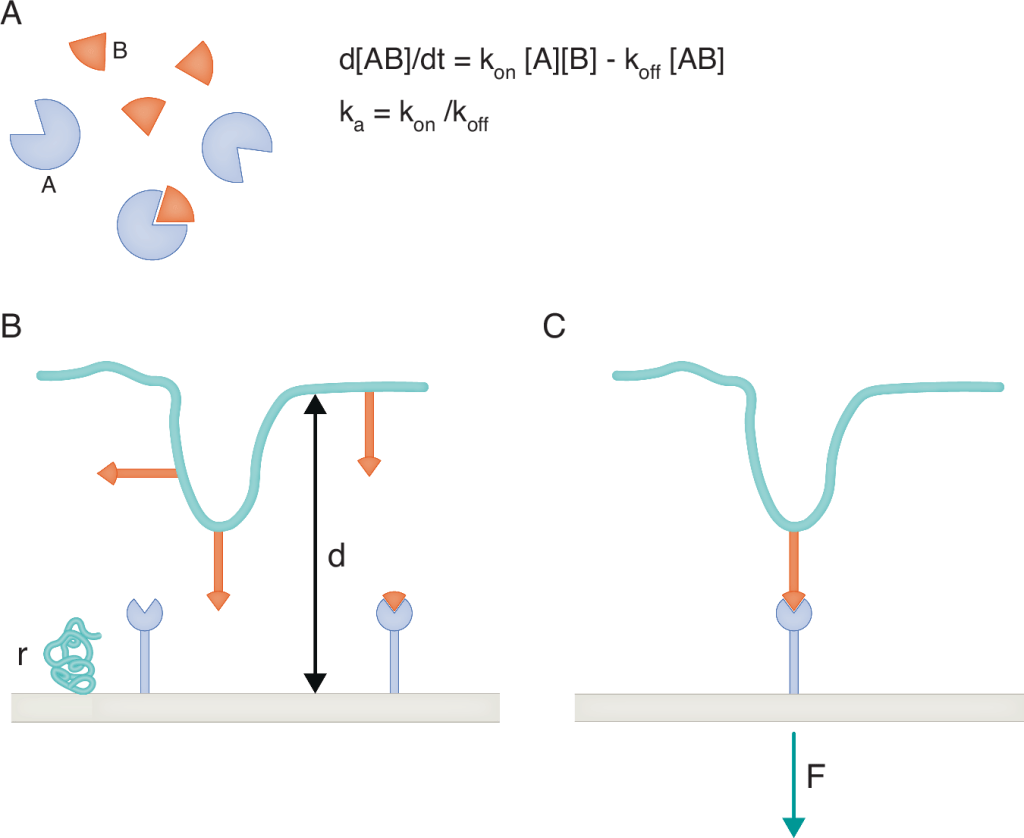
Figure 1. Difficulty of comparing 2D and 3D molecule association.
(A) Conventional 3D molecular binding. Molecular encounters are well-described by standard diffusion equations. Molecular translation and rotation are weakly dependent on molecular size and shape. Bond rupture is driven by thermal fluctuations. The association rate (kon), dissociation rate (koff), and affinity constant (Ka) are thus determined by molecular structure and generic mechanisms, they may thus be considered as intrinsic properties of interacting molecules A and B. (B) 2D association. When ligand and receptor molecules are bound to surfaces, the frequency of encounters is strongly dependent on the distance d beteween surfaces, surface roughness, flexibility of the anchors of binding sites that drive molecular contact frequency and relative molecule orientation, lateral diffusion of anchored molecules, and possible role of bulk repeller molecules (r) on surfaces. (C) 2D dissociation. Bond rupture is strongly dependent on the intensity and time-dependence of forces exerted on the bonds. When a bond is broken, the dynamics of anchoring surfaces may influence rebinding.




















You must be logged in to post a comment.