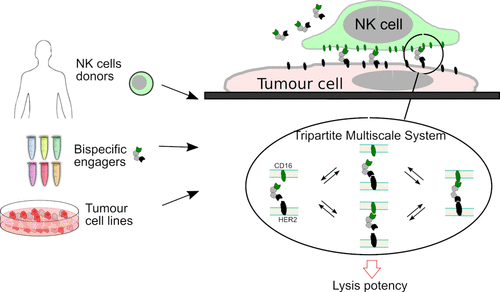
In recent years, immunotherapy has brought about a paradigm shift in the treatment of cancer, making it possible to stimulate and re-arm the immune system against the disease. One strategy already used against certain cancers involves injecting molecular engaging agents (e.g. bispecific monoclonal antibodies) capable of recruiting immune cells such as lymphocytes to directly attack the targeted tumour cells. These bispecific molecules act as a molecular bridge between activating receptors on immune cells and tumour antigens exposed by the target cells. Despite the potential of these new agents, the ability to predict their efficacy remains limited, as it depends on both the patient and the details of the agents molecular structure. Patrick Chames and his team (CRCM) have for several years been developing bispecific antibodies constructed from camelid monodomain antibodies, known as nanobodies.
the authors tested in-vitro a range of 6 original bispecific antibodies combining several nanobodies, and recruiting Natural Killer cells from 15 healthy donors to different target tumour cell lines. They systematically measured the quantity of target cells killed for varying concentrations of engaging molecules, taking into account biophysical parameters such as antibody affinity and receptor density. These data showed that it was possible to largely decouple the role of donor cells from the molecular details of the engager to predict the effective engager dose. They also proposed a simple formula for quantitatively predicting this effective dose in vitro.

You must be logged in to post a comment.