Binding of the T cell receptor complex to its ligand, the subsequent molecular rearrangement, and the concomitant cell-scale shape changes represent the very first steps of adaptive immune recognition. The first minutes of the interaction of T cells and antigen presenting cells have been extensively scrutinized; yet, gaps remain in our understanding of how the biophysical properties of the environment may impact the sequence of events. In particular, many pioneering experiments were done on immobilized ligands and gave major insights into the process of T cell activation, whereas later experiments have indicated that ligand mobility was of paramount importance, especially to enable the formation of T cell receptor clusters. Systematic experiments to compare and reconcile the two schools are still lacking. Furthermore, recent investigations using compliant substrates have elucidated other intriguing aspects of T cell mechanics. Here we review experiments on interaction of T cells with planar artificial antigen presenting cells to explore the impact of mechanics on adhesion and actin morphodynamics during the spreading process. We enumerate a sequence tracing first contact to final spread state that is consistent with current understanding. Finally, we interpret the presented experimental results in light of a mechanical model that captures all the different morphodynamic states.
https://doi.org/10.1016/j.bpj.2024.02.023
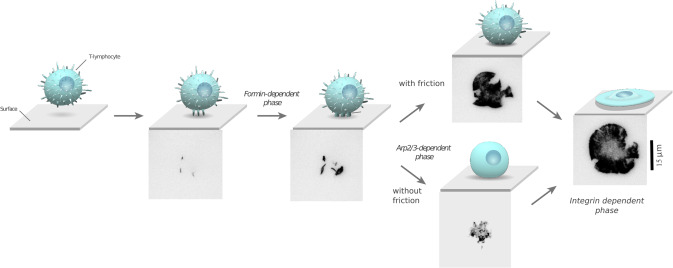
Sequence of ligand recognition and spreading phases. Schemes (not to scale) show T cell morphology, and micrographs show labeled actin cytoskeleton (GFP-lifeact, TIRF mode) as a T cell undergoes adhesion to a ligand-covered surface.






You must be logged in to post a comment.