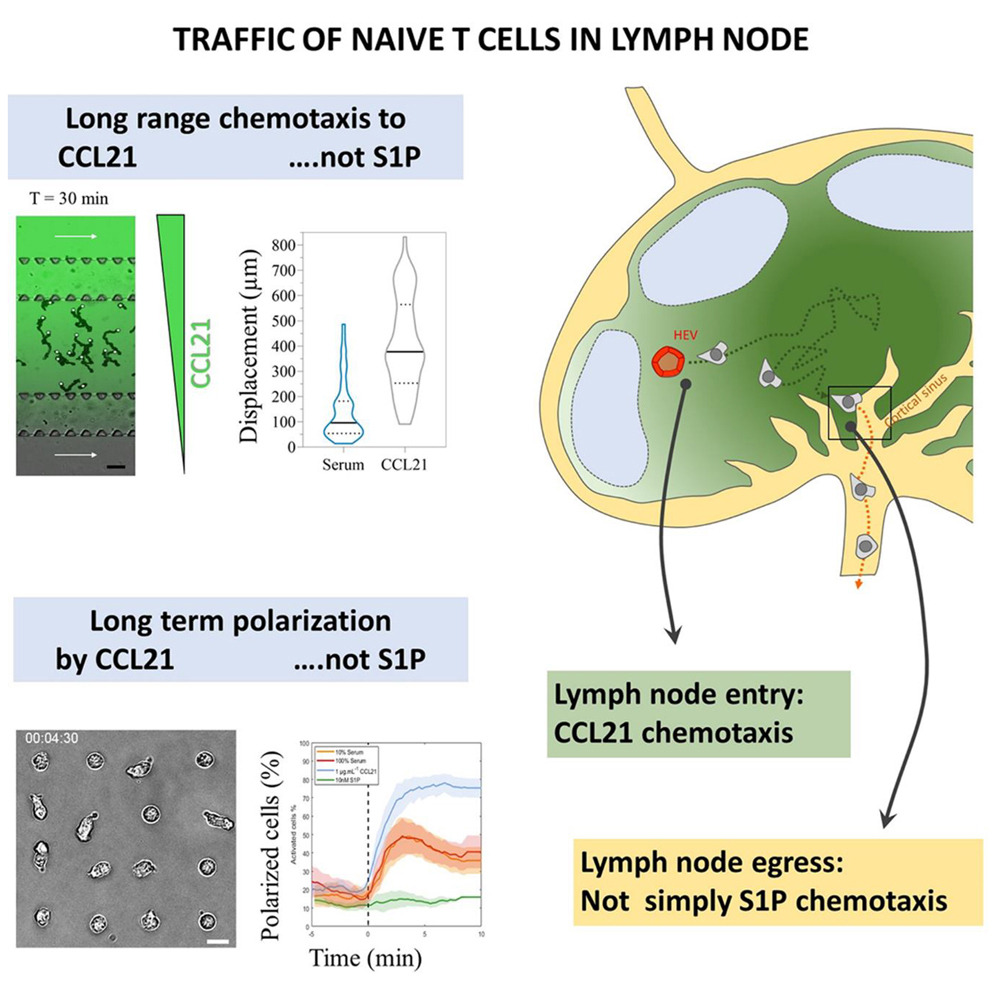
Optical tweezers are a light-based technique for micromanipulating objects. It allows to move objects such as microbeads and cells, and to record minute forces down to a few pN, which makes it a technique very well adapted to mechanical measurements on living cells (Gennerich, 2017). We are interested in the mechanotransduction properties of lymphocytes. We seek to dissect the effect of forces and cell mechanics on the cellular response, in the context of the immune system. T cell mechanotransduction has been recently demonstrated to be instrumental in the finesse and accuracy of the response of the latter Puech & Bongrand (2021)]. In addition, cells can exert forces when performing their action, e.g. cytotoxic T cells are using forces to kill target infected cells (Basu et al., 2016).
Using optical tweezers and specifically decorated beads as handles, we pull membrane nano- tubes from gently adhered living lymphocytes (Sadoun et al., 2020). Such nanotubes are usually used to probe the tension of adherent cells (Diz-Muñoz et al., 2010). By varying the antibodies that are used to decorate the beads, we select the molecule type we specifically pull on, and we then explore the molecules which are characteristic of the immune synapse, which is one of the key organizational structures that have profound implications in T cell recognition and action (Baldari & Dustin, 2017).
Using this approach, we probe not only the forces of recognition of the given antibody to its target molecule, but also, by using strong extracellular bridges, we probe the cytosolic link of the probed molecule to the cytoskeleton. Such a link has been proposed to be instrumental in the way T cells can apply or feel forces through the molecule. A theoretical model has been built and has been recently reported in a dedicated article (Manca et al., 2023). Furthermore, we will demonstrate the application of the software on full data.
The experimentally obtained data consists of force signal as a function of time (among other parameters), in the three directions of space, obtained in large quantities (at least 10 per cell / bead couple, and up to 20 couples tested per sample), containing rich and detailed features that can relate to molecular and/or cellular mechanics that our model explores. It is therefore needed to standardize and semi-automate data analysis to help the experimentalist, often a biologist, to extract relevant features from the experimental data sets.
https://doi.org/10.21105/joss.04877








You must be logged in to post a comment.